An outdated antibiotic might present much-needed safety towards multi-drug resistant bacterial infections, in keeping with a brand new research revealed Could 16th within the open entry journal by James Kirby of Harvard Medical College, US, and colleagues. The discovering might supply a brand new method to struggle difficult-to-treat and doubtlessly deadly infections.
Examine: . Picture Credit score: Kateryna Kon / Shutterstock
Nourseothricin is a pure product made by a soil fungus, which comprises a number of types of a fancy molecule known as streptothricin. Its discovery within the Nineteen Forties generated excessive hopes for it as a robust agent towards Gram-negative micro organism, which, on account of their thick outer protecting layer, are particularly arduous to kill with different antibiotics. However nourseothricin proved poisonous to kidneys, and its growth was dropped. Nevertheless, the rise of antibiotic-resistant bacterial infections has spurred the seek for new antibiotics, main Kirby and colleagues to take one other have a look at nourseothricin.
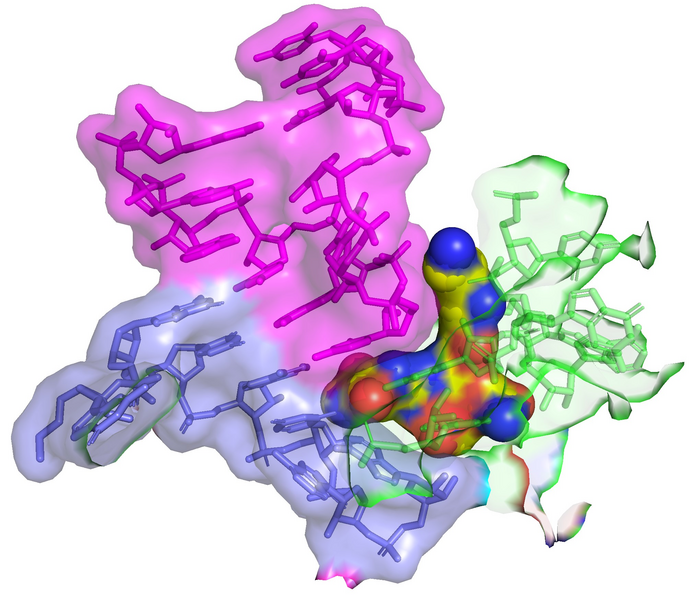
Streptothricin-F (yellow spheres) sure to the 16S rRNA (inexperienced) of the bacterial ribosome impinges on the decoding website the place tRNA (purple) binds to the codon of the mRNA (blue). This interplay results in translation infidelity (scrambled protein sequences) and the ensuing dying of the bacterial cell. The picture was created by overlay of PDB 7UVX containing streptothricin-F (this manuscript) with PDB 7K00 containing mRNA and A-site tRNA (ref DOI: 10.7554/eLife.60482). Picture Credit score: James Kirby (CC-BY 4.0, ); Zoe L Watson et al., 2023, eLife, CC-BY 4.0 ()
Early research of nourseothricin suffered from incomplete purification of the streptothricins. Extra current work has proven that the a number of varieties have completely different toxicities, with one, streptothricin-F, considerably much less poisonous whereas remaining extremely lively towards modern multidrug-resistant pathogens. Right here, the authors characterised the antibacterial motion, renal toxicity, and mechanism of motion of extremely purified types of two completely different streptothricins, D and F. The D type was stronger than the F type towards drug-resistant Enterobacterales and different bacterial species however prompted renal toxicity at a decrease dose. Each had been extremely selective for Gram-negative micro organism.
Utilizing cryo-electron microscopy, the authors confirmed that streptothricin-F sure extensively to a subunit of the bacterial ribosome, accounting for the interpretation errors these antibiotics are identified to induce of their goal micro organism. Curiously, the binding interplay is distinct from different identified inhibitors of translation, suggesting it might discover use when these brokers should not efficient.
“Based mostly on distinctive, promising exercise,” Kirby mentioned, “we imagine the streptothricin scaffold deserves additional pre-clinical exploration as a possible therapeutic for the remedy of multidrug-resistant, Gram-negative pathogens.”
Kirby provides, “Remoted in 1942, streptothricin was the primary antibiotic found with potent gram-negative exercise. We discover that not solely is it exercise potent, however that it’s extremely lively the hardiest modern multidrug-resistant pathogens and works by a novel mechanism to inhibition protein synthesis.”
Supply:
Journal reference: